Sex differences in winter distribution of Long-eared Owls (Asio otus)
in Denmark and neighbouring countries
By Johannes Erritzoe & Richard Fuller
Copyright: Johannes Erritzoe & Richard Fuller, 1999
 Abstract:
Johannes Erritzoe and Richard Fuller (1998): Sex differences in winter
distribution of Long-eared Owls (Asio otus) in Denmark and neighbouring
countries. Vogelwarte 40: 80-87. Abstract:
Johannes Erritzoe and Richard Fuller (1998): Sex differences in winter
distribution of Long-eared Owls (Asio otus) in Denmark and neighbouring
countries. Vogelwarte 40: 80-87.
Evidence presented here indicates that in western Denmark, the sex
ratio of Long-eared Owls (Asio otus) during the winter months is significantly
biased towards females. Museum collections revealed the same phenomenon
in the Netherlands and northern Germany, while sex ratios approached
parity in eastern Denmark, Sweden and central and southern Germany.
From the literature, it is clear that there is also a significant winter
surplus of females in England, Scotland and the Shetland Islands. Present
hypotheses for differential migration are reviewed and it is concluded
that none explain adequately this pattern. An alternative hypothesis
is presented here, in which heavy predation pressure by Eagle Owls (Bubo
bubo) and Goshawks (Accipiter gentilis) on the breeding range in Fenno-Scandia
and parts of these birds' wintering range in northern and western Europe
is the primary cause of the differential in the observed pattern of
winter distribution.
 Key
words: Long-eared Owl (Asio otus), sex ratio, differential
migration, Eagle Owl (Bubo bubo), Goshawk (Accipiter gentilis). Key
words: Long-eared Owl (Asio otus), sex ratio, differential
migration, Eagle Owl (Bubo bubo), Goshawk (Accipiter gentilis).
Addresses:
Johannes Eritzoe
House of Bird Research,
Taps Old Rectory,
DK-6070 Christiansfeld,
Denmark
E-mail: erritzoe@birdresearch.dk
Richard Fuller
33 Plough Road,
West Ewell,
Surrey,
KT19 9RA,
UK.
E-mail: Richard@fuller83.freeserve.co.uk
1. Introduction
 Partial
migration is a behavioural phenomenon that varies with respect to time,
geographical distribution, sex, and age (Berthold 1984). Food shortage
in winter seems to be the main stimulus for migration among birds, and
if there is not food enough for both sexes, the general pattern is for
the dominant sex to remain on or near the breeding grounds and the subordinate
sex to migrate (e.g. Lack 1966, Gauthreaux 1978, Lundberg 1988). Unlike
most owls, female Long-eared Owls (Asio otus) show a greater
migratory tendency than do males, the hole-nesting Tengmalm's Owl (Aegolius
funereus) being the only other exception in Europe (Lundberg 1979).
Female Long-eared Owls are larger than males, with a body mass on average
15-17% greater, although female wing length is only 1% to 1.7% larger,
with much overlap (Glutz von Blotzheim & Bauer 1980, Cramp 1985,
Lundberg 1986). In spite of the large difference in mass, sexual dimorphism
in Long-eared Owls is not obvious in the field and generally is only
appreciable with the bird in the hand. Partial
migration is a behavioural phenomenon that varies with respect to time,
geographical distribution, sex, and age (Berthold 1984). Food shortage
in winter seems to be the main stimulus for migration among birds, and
if there is not food enough for both sexes, the general pattern is for
the dominant sex to remain on or near the breeding grounds and the subordinate
sex to migrate (e.g. Lack 1966, Gauthreaux 1978, Lundberg 1988). Unlike
most owls, female Long-eared Owls (Asio otus) show a greater
migratory tendency than do males, the hole-nesting Tengmalm's Owl (Aegolius
funereus) being the only other exception in Europe (Lundberg 1979).
Female Long-eared Owls are larger than males, with a body mass on average
15-17% greater, although female wing length is only 1% to 1.7% larger,
with much overlap (Glutz von Blotzheim & Bauer 1980, Cramp 1985,
Lundberg 1986). In spite of the large difference in mass, sexual dimorphism
in Long-eared Owls is not obvious in the field and generally is only
appreciable with the bird in the hand.
 There
is considerable and confusing variation in sex ratio among migrating
and wintering Long-eared Owl populations in Europe. Harvey & Riddiford
(1990) found 28 females and 8 males among autumn migrant Long-eared
Owls ringed on Fair Isle, Shetland, and among 123 dead Long-eared Owls
from England and Scotland received at the Institute of Terrestrial Ecology
during 1963-1995, there was a ratio of 89 females : 34 males (Wyllie
et al. 1996). In birds killed between October and February, the sex
ratio was even more strongly female biased (42 females : 8 males), whereas
between May and September, the ratio was close to parity (12 females
: 10 males). In the Netherlands, Wijnandts (1984) found a sex ratio
among traffic casualties of 17 females : 8 males. Further north, the
situation is very different. An examination of traffic victims collected
by the Norwegian Institute for Nature Research during 1987 - 1992 revealed
a winter sex ratio of 13 females : 23 males (Overskaug & Kristiansen
1994), a pattern already noted in Finland many years ago (Hortling 1929
p. 356). Most migrant Long-eared Owls are first year birds e.g. on Heligoland,
of 79 birds ringed in October and November, 74 were birds of the year,
some still with down on their feather tips (Moritz & Schonart 1976). There
is considerable and confusing variation in sex ratio among migrating
and wintering Long-eared Owl populations in Europe. Harvey & Riddiford
(1990) found 28 females and 8 males among autumn migrant Long-eared
Owls ringed on Fair Isle, Shetland, and among 123 dead Long-eared Owls
from England and Scotland received at the Institute of Terrestrial Ecology
during 1963-1995, there was a ratio of 89 females : 34 males (Wyllie
et al. 1996). In birds killed between October and February, the sex
ratio was even more strongly female biased (42 females : 8 males), whereas
between May and September, the ratio was close to parity (12 females
: 10 males). In the Netherlands, Wijnandts (1984) found a sex ratio
among traffic casualties of 17 females : 8 males. Further north, the
situation is very different. An examination of traffic victims collected
by the Norwegian Institute for Nature Research during 1987 - 1992 revealed
a winter sex ratio of 13 females : 23 males (Overskaug & Kristiansen
1994), a pattern already noted in Finland many years ago (Hortling 1929
p. 356). Most migrant Long-eared Owls are first year birds e.g. on Heligoland,
of 79 birds ringed in October and November, 74 were birds of the year,
some still with down on their feather tips (Moritz & Schonart 1976).
 It
therefore seems clear that sex ratios in wintering Long-eared Owl populations
vary geographically, some areas showing a strongly female-biased sex-ratio,
others exhibiting near parity. Which environmental factors or other
selective pressures may have given rise to this marked intersexual difference
in migration strategy and the complex pattern of winter female distribution? It
therefore seems clear that sex ratios in wintering Long-eared Owl populations
vary geographically, some areas showing a strongly female-biased sex-ratio,
others exhibiting near parity. Which environmental factors or other
selective pressures may have given rise to this marked intersexual difference
in migration strategy and the complex pattern of winter female distribution?
 As
patterns of movement and distribution outside the breeding season are
often influenced by breeding behaviour and intersexual role allocation
(Myers 1981), a few basic biological data are necessary. The Long-eared
Owl is a Holarctic species, in the Palaearctic found from western Europe
to Japan and China (Mikkola 1983). The breeding season in Denmark is
from early March to May (Olsen 1992). Males defend what is often only
a very small territory, for instance three pairs were found breeding
in an area little over two hectares in Denmark (Trap-Lind 1965, Glutz
von Blotzheim & Bauer 1980, Cramp 1985). Disused Carrion and Hooded
Crow (Corvus corone) or Common Magpie (Pica pica) nests are favoured;
females incubate and rear the nestlings, while males bring food to both
the young and the female (Mikkola 1983). As
patterns of movement and distribution outside the breeding season are
often influenced by breeding behaviour and intersexual role allocation
(Myers 1981), a few basic biological data are necessary. The Long-eared
Owl is a Holarctic species, in the Palaearctic found from western Europe
to Japan and China (Mikkola 1983). The breeding season in Denmark is
from early March to May (Olsen 1992). Males defend what is often only
a very small territory, for instance three pairs were found breeding
in an area little over two hectares in Denmark (Trap-Lind 1965, Glutz
von Blotzheim & Bauer 1980, Cramp 1985). Disused Carrion and Hooded
Crow (Corvus corone) or Common Magpie (Pica pica) nests are favoured;
females incubate and rear the nestlings, while males bring food to both
the young and the female (Mikkola 1983).
 Most
Long-eared Owls stay on their breeding grounds throughout the year when
food supply is adequate, but in years with fewer prey, large numbers
of owls move from Fenno-Scandia to central and western Europe (Glutz
von Blotzheim & Bauer 1980). In Finland, about 80% of all birds
are nomadic and only breed when and where food is abundant (Juvonen
1976 cited in Cramp 1985). This is consistent with the results of a
theoretical analysis predicting that partial migration will be most
frequent in regions where climatic variability is high (Cohen 1967,
cited in Lundberg 1988). These data also support the hypothesis that
predicts an increasing proportion of partially migratory birds with
increasing latitude (Lundberg 1988). Most
Long-eared Owls stay on their breeding grounds throughout the year when
food supply is adequate, but in years with fewer prey, large numbers
of owls move from Fenno-Scandia to central and western Europe (Glutz
von Blotzheim & Bauer 1980). In Finland, about 80% of all birds
are nomadic and only breed when and where food is abundant (Juvonen
1976 cited in Cramp 1985). This is consistent with the results of a
theoretical analysis predicting that partial migration will be most
frequent in regions where climatic variability is high (Cohen 1967,
cited in Lundberg 1988). These data also support the hypothesis that
predicts an increasing proportion of partially migratory birds with
increasing latitude (Lundberg 1988).
Differential migration hypotheses
 Various
hypotheses for the latitudinal segregation of sexes in winter have been
presented (review in Clark et al. 1978, Myers 1981, Ketterson &
Nolan 1983, Berthold 1984, Kjellén 1994 ). Below is brief summary
of each of the main hypotheses, with comments regarding the applicability
of each to the Long-eared Owl system as described above: Various
hypotheses for the latitudinal segregation of sexes in winter have been
presented (review in Clark et al. 1978, Myers 1981, Ketterson &
Nolan 1983, Berthold 1984, Kjellén 1994 ). Below is brief summary
of each of the main hypotheses, with comments regarding the applicability
of each to the Long-eared Owl system as described above:
(1) Intersexual competition for food in winter impels the smaller,
subordinate sex to migrate (Gauthreaux 1978, Ketterson 1979. Ketterson
& Nolan 1979).
 This
hypothesis is not supported by the Long-eared Owl since the much larger
female migrates in the winter months from parts of Fenno-Scandia and
Denmark to England and other parts of northern Europe, while the smaller,
subordinate male remains nearer the breeding grounds. However, food
shortage in winter probably is the major factor controlling ultimate
densities of temperate bird populations (Lack 1966), here confirmed
by a greater immigration to England and Denmark in years with low vole
populations further north (cf. Wyllie et al. 1996, pers obs.). This
hypothesis is not supported by the Long-eared Owl since the much larger
female migrates in the winter months from parts of Fenno-Scandia and
Denmark to England and other parts of northern Europe, while the smaller,
subordinate male remains nearer the breeding grounds. However, food
shortage in winter probably is the major factor controlling ultimate
densities of temperate bird populations (Lack 1966), here confirmed
by a greater immigration to England and Denmark in years with low vole
populations further north (cf. Wyllie et al. 1996, pers obs.).
(2) If there is competition for breeding resources, it is an advantage
for the individuals of one sex, usually males, to winter closer to the
breeding grounds, because they can arrive earlier in the breeding season
(Ketterson & Nolan 1976).
 There
is no evidence for strong territoriality or competition for breeding
resources in the Long-eared Owl; there are very few reports of aggressive
encounters and the species is well known for its communal roosting behaviour
in winter (Cramp 1985). In view of the fact that nests are regularly
placed very close together, and the lack of apparent territoriality
in this species, this hypothesis is not supported by the pattern of
differential migration exhibited by Long-eared Owls. There
is no evidence for strong territoriality or competition for breeding
resources in the Long-eared Owl; there are very few reports of aggressive
encounters and the species is well known for its communal roosting behaviour
in winter (Cramp 1985). In view of the fact that nests are regularly
placed very close together, and the lack of apparent territoriality
in this species, this hypothesis is not supported by the pattern of
differential migration exhibited by Long-eared Owls.
(3) Physiological limitations related to body size (greater cold
tolerance and ability to fast) permit the larger sex to winter in harsher
climates (Ketterson & Nolan 1976, Ketterson & King 1977, Ketterson
& Nolan 1979, cf. Bergman´s rule).
 Bergmann´s
rule predicts that birds of the same species or group of species are
larger at higher than lower latitudes, because birds in colder climates
need greater volume to area relations to maintain body temperature.
The Long-eared Owl is a clear exception to this rule and the data do
not support this hypothesis. Bergmann´s
rule predicts that birds of the same species or group of species are
larger at higher than lower latitudes, because birds in colder climates
need greater volume to area relations to maintain body temperature.
The Long-eared Owl is a clear exception to this rule and the data do
not support this hypothesis.
(4) Risk of mortality on migration may vary intersexually, leading
to one sex becoming sedentary while the other is migratory (Ketterson
& Nolan 1983).
 That
migration carries a high risk of mortality, the generally accepted rule
for migrating species, seems also to apply to the Long-eared Owl in
normal years. Finnish migrants of all ages, except first calendar year
birds, exhibit a higher mortality rate than their non-migrating conspecifics
(Finnish recovery printouts). If mortality risk on migration is indeed
different between sexes in the Long-eared Owl, the larger and less manoeuvrable
female is likely to be at greater risk, particularly in relation to
traffic accidents, and therefore less likely to migrate than the male.
This hypothesis can also be rejected. That
migration carries a high risk of mortality, the generally accepted rule
for migrating species, seems also to apply to the Long-eared Owl in
normal years. Finnish migrants of all ages, except first calendar year
birds, exhibit a higher mortality rate than their non-migrating conspecifics
(Finnish recovery printouts). If mortality risk on migration is indeed
different between sexes in the Long-eared Owl, the larger and less manoeuvrable
female is likely to be at greater risk, particularly in relation to
traffic accidents, and therefore less likely to migrate than the male.
This hypothesis can also be rejected.
 None
of the existing hypotheses seems adequately to explain the pattern of
differential migration found in the Long-eared Owl. This study will
use sex-ratio data from European Long-eared Owl wintering populations
to examine more closely the distribution of the sexes in winter and
seek a suitable explanation. None
of the existing hypotheses seems adequately to explain the pattern of
differential migration found in the Long-eared Owl. This study will
use sex-ratio data from European Long-eared Owl wintering populations
to examine more closely the distribution of the sexes in winter and
seek a suitable explanation.
2. Material and methods
 New
information is here presented on sex-ratios of accident victims among
Long-eared Owls in Denmark based on eleven years of study, and of birds
in England, Germany, the Netherlands and Sweden through study of museum
material from the larger collections in northern Europe. New
information is here presented on sex-ratios of accident victims among
Long-eared Owls in Denmark based on eleven years of study, and of birds
in England, Germany, the Netherlands and Sweden through study of museum
material from the larger collections in northern Europe.
 As
winter is the critical period for testing sex-ratio with respect to
partial migration, specimens taken during breeding months were eliminated.
Any gross intersexual difference in winter detectability through differential
mortality was considered unlikely, given that both sexes hunt in winter,
unlike during the breeding season when mainly the male hunts (e.g. Mikkola
1983, Cramp 1985). All Long-eared Owls brought to JE in Taps, Jutland,
Denmark, between 1 November and the end of February over an eleven
year period (1986 - 1997) were examined by gonadal inspection and aged
using the criteria described in Piechocki (1979). In addition to sex
and age, the date, locality and cause of death were recorded for each
specimen. A total of 45 owls was studied by JE, with two additional
birds examined by Hugo Christensen. The same information was collected
from skins taken between November and February in Denmark housed in
the Zoologisk Museum in Copenhagen (n = 30) and Naturhistorisk Museum
in Århus (n = 5). This amounts to a total of 82 birds examined
in detail. In addition, winter sex ratios of Long-eared Owls in England,
Germany, the Netherlands and Sweden were calculated, using the collections
of native birds in the Museums of Natural History in Tring, Berlin,
Bonn, Frankfurt, Dresden, Halberstadt, Wuppertal, Leiden, Stockholn,
and Malmö. Data on body mass of Danish owls examined by JE were
taken, and body masses of Swedish owls were collected from specimen
labels in the Malmö collection. As
winter is the critical period for testing sex-ratio with respect to
partial migration, specimens taken during breeding months were eliminated.
Any gross intersexual difference in winter detectability through differential
mortality was considered unlikely, given that both sexes hunt in winter,
unlike during the breeding season when mainly the male hunts (e.g. Mikkola
1983, Cramp 1985). All Long-eared Owls brought to JE in Taps, Jutland,
Denmark, between 1 November and the end of February over an eleven
year period (1986 - 1997) were examined by gonadal inspection and aged
using the criteria described in Piechocki (1979). In addition to sex
and age, the date, locality and cause of death were recorded for each
specimen. A total of 45 owls was studied by JE, with two additional
birds examined by Hugo Christensen. The same information was collected
from skins taken between November and February in Denmark housed in
the Zoologisk Museum in Copenhagen (n = 30) and Naturhistorisk Museum
in Århus (n = 5). This amounts to a total of 82 birds examined
in detail. In addition, winter sex ratios of Long-eared Owls in England,
Germany, the Netherlands and Sweden were calculated, using the collections
of native birds in the Museums of Natural History in Tring, Berlin,
Bonn, Frankfurt, Dresden, Halberstadt, Wuppertal, Leiden, Stockholn,
and Malmö. Data on body mass of Danish owls examined by JE were
taken, and body masses of Swedish owls were collected from specimen
labels in the Malmö collection.
 We
are grateful to all who have forwarded specimens (Dr Jon Fjeldså,
Marius Nørgård, Thorkil Duch and Hugo Christensen). We
wish to express our sincere thanks for hospitality and assistance to
all the staff at the museums visited in Århus, Berlin, Copenhagen,
Leiden and Tring (Dr Anders Holm Joensen, Erling Mørch, Dr B.
Stephan, Dr Jon Fjeldså, Dr René Dekker, Dr Robert Prys-Jones
and Michael P. Walters). The following have sent complete lists of their
museum holdings of Long-eared Owls, for which we acknowledge our indebtedness:
Siegfried Eck, Göran Frisk, Thomas Gütebier, Dr W. Kolbe,
Dr B. Nicolai, Dr D. S. Peters and Dr Renate van den Elzen. We are very
grateful to Prof. Anders Pape Møller for valuable comments on
the draft. We
are grateful to all who have forwarded specimens (Dr Jon Fjeldså,
Marius Nørgård, Thorkil Duch and Hugo Christensen). We
wish to express our sincere thanks for hospitality and assistance to
all the staff at the museums visited in Århus, Berlin, Copenhagen,
Leiden and Tring (Dr Anders Holm Joensen, Erling Mørch, Dr B.
Stephan, Dr Jon Fjeldså, Dr René Dekker, Dr Robert Prys-Jones
and Michael P. Walters). The following have sent complete lists of their
museum holdings of Long-eared Owls, for which we acknowledge our indebtedness:
Siegfried Eck, Göran Frisk, Thomas Gütebier, Dr W. Kolbe,
Dr B. Nicolai, Dr D. S. Peters and Dr Renate van den Elzen. We are very
grateful to Prof. Anders Pape Møller for valuable comments on
the draft.
3. Results
 Data
on Long-eared Owl winter sex ratios are presented in Table 1. In all
cases we used a G test with the appropriate Williams' correction (Fowler
& Cohen no date) to examine the data for significant relationships.
The sex ratio amongst wintering owls in western Denmark (Jutland and
Funen) was strongly female-biased (48 females : 10 males, Gadj=26.4,
p<0.01), whereas eastern Danish populations (Zealand, Falster, Anholt
and Bornholm) exhibited parity. The difference between these populations
was highly significant (Gadj=8.51, p<0.01). The sexes and locations
of the 82 Danish specimens are mapped in figure 1. JE resides in southern
Jutland, hence the concentration of records there. Data
on Long-eared Owl winter sex ratios are presented in Table 1. In all
cases we used a G test with the appropriate Williams' correction (Fowler
& Cohen no date) to examine the data for significant relationships.
The sex ratio amongst wintering owls in western Denmark (Jutland and
Funen) was strongly female-biased (48 females : 10 males, Gadj=26.4,
p<0.01), whereas eastern Danish populations (Zealand, Falster, Anholt
and Bornholm) exhibited parity. The difference between these populations
was highly significant (Gadj=8.51, p<0.01). The sexes and locations
of the 82 Danish specimens are mapped in figure 1. JE resides in southern
Jutland, hence the concentration of records there.
 Like
eastern Danish populations, Swedish owls exhibited near parity (132
females : 135 males, Gadj=0.03, p>0.05). A similar result was found
for Germany as a whole (46 females : 38 males, Gadj=0.75, p>0.05),
although a significant female bias was found among owls collected in
the north of the country (15 females : 5 males, Gadj=4.87, p<0.05).
Owls from the Netherlands also demostrated a female-biased sex ratio
(27 females : 10 males, Gadj=7.8, p<0.01), as did UK birds, although
sample sizes of the latter were too small for statistical significance
(9 females : 4 males, Gadj=1.77, p>0.05). Like
eastern Danish populations, Swedish owls exhibited near parity (132
females : 135 males, Gadj=0.03, p>0.05). A similar result was found
for Germany as a whole (46 females : 38 males, Gadj=0.75, p>0.05),
although a significant female bias was found among owls collected in
the north of the country (15 females : 5 males, Gadj=4.87, p<0.05).
Owls from the Netherlands also demostrated a female-biased sex ratio
(27 females : 10 males, Gadj=7.8, p<0.01), as did UK birds, although
sample sizes of the latter were too small for statistical significance
(9 females : 4 males, Gadj=1.77, p>0.05).
|
|
|
|
|
Stockholm (RMS)
|
|
|
|
Malmoe (MM)
|
|
|
|
Eastern Denmark (Zealand, Falster, Anholt and Bornholm)
|
|
|
|
Western Denmark (Jutland and Funen)
|
|
|
|
Northeastern Germany
|
|
|
|
Eastern Germany
|
|
|
|
Western Germany
|
|
|
|
Southern Germany
|
|
|
|
Netherlands
|
|
|
|
Tring (BMNH)
|
|
|
|
* Sex ratio significantly female biased at the 5% level (p<0.05,
G test)
|
|
** * Sex ratio significantly female biased at the 1% level (p<0.01,
G test)
|
Table 1: Sex-ratio of Long-eared Owls from Germany,
the Netherlands, England, Sweden and Denmark. Data are arranged
in geographical order from northeast to southwest.
Tab. 1: Verhältnis der Geschlechter der Waldohreulen
in Deutschland, Holland, England, Schweden und Dänemark. Die Daten
sind geographish von Nordost bis Südwest aufgelistet.
|
 Body
mass data are presented in table 2. Note the larger mass dimorphism
(16.9%) in the Netherlands than in Sweden (12.4%) and Denmark (11.0%). Body
mass data are presented in table 2. Note the larger mass dimorphism
(16.9%) in the Netherlands than in Sweden (12.4%) and Denmark (11.0%).
4. Discussion
 As
described above, none of the existing hypotheses accounts well for the
reverse migration patterns observed in the Long-eared Owl. On the basis
of the results of our study, we suggest a new hypothesis: Where a
sexually dimorphic population is under heavy predation pressure, the
more vulnerable sex is more likely to migrate in winter to areas without
or with fewer of the main predator(s). As
described above, none of the existing hypotheses accounts well for the
reverse migration patterns observed in the Long-eared Owl. On the basis
of the results of our study, we suggest a new hypothesis: Where a
sexually dimorphic population is under heavy predation pressure, the
more vulnerable sex is more likely to migrate in winter to areas without
or with fewer of the main predator(s).
 A
source of possible predation pressure on the Long-eared Owl is the Eagle
Owl Bubo bubo, sympatric with the Long-eared Owl in large areas of Norway,
Sweden, Finland, Germany and Russia (Glutz von Blotzheim & Bauer
1980, Cramp 1985, Hagemeijer & Blair 1997). According to Tucker
and Heath (1994), the breeding population in Norway is between 1,000
and 3,000 pairs, Sweden 250 - 350, Finland 2,000 - 3,000, Germany 400
- 500, Russia 2,000 - 20,000, Netherlands 0 - 2, Luxenburg 1 - 5 and
England 0. In Denmark the population is currently estimated at 50 pairs,
thanks to recent immigration from Germany and Sweden (Klaus Dickman
pers. comm.). Eagle Owls hunt essentially nocturnally and take other
owl species more often than their availability warrants (Mikkola 1983,
Cramp 1985) . Mikkola (1983) recorded predation events involving owls
in Europe and found that the Long-eared Owl was the most numerous owl
prey of the Eagle Owl (118 records). Uttendörfer (1939) studied
118,000 German bird remains taken by birds of prey and owls and found
155 Long-eared Owls killed by Goshawks (Accipiter gentilis) and
62 Long-eared Owls killed by Eagle Owls. In northern Bavaria, Germany,
3.7 % of all prey taken by Eagle Owls during the breeding season were
Long-eared Owls (Bezzel et al. 1976), and in northeastern Harz Foreland,
21 Long-eared Owls were found among 612 bird-prey items in pellets of
8-10 breeding pairs of Eagle Owls (Wadewitz & Nicolai 1993). Among
1048 Long-eared Owl ringing recoveries from Finland, Sweden and Russia,
23 were killed by owls or raptors and a further 45 were predated by
unknown animals (ringing recovery printouts from Sweden, Finland and
Russia). Eagle Owls and Goshawks therefore seem to be important predators
of the Long-eared Owl and as such, the degree of predator pressure may
contribute to the pattern of differential migration seen in the last
species. As the female is heavier but without a correspondingly greater
wingspan, it is presumably easier prey for the Eagle Owl and the Goshawk
than the more manoeuvrable male. A
source of possible predation pressure on the Long-eared Owl is the Eagle
Owl Bubo bubo, sympatric with the Long-eared Owl in large areas of Norway,
Sweden, Finland, Germany and Russia (Glutz von Blotzheim & Bauer
1980, Cramp 1985, Hagemeijer & Blair 1997). According to Tucker
and Heath (1994), the breeding population in Norway is between 1,000
and 3,000 pairs, Sweden 250 - 350, Finland 2,000 - 3,000, Germany 400
- 500, Russia 2,000 - 20,000, Netherlands 0 - 2, Luxenburg 1 - 5 and
England 0. In Denmark the population is currently estimated at 50 pairs,
thanks to recent immigration from Germany and Sweden (Klaus Dickman
pers. comm.). Eagle Owls hunt essentially nocturnally and take other
owl species more often than their availability warrants (Mikkola 1983,
Cramp 1985) . Mikkola (1983) recorded predation events involving owls
in Europe and found that the Long-eared Owl was the most numerous owl
prey of the Eagle Owl (118 records). Uttendörfer (1939) studied
118,000 German bird remains taken by birds of prey and owls and found
155 Long-eared Owls killed by Goshawks (Accipiter gentilis) and
62 Long-eared Owls killed by Eagle Owls. In northern Bavaria, Germany,
3.7 % of all prey taken by Eagle Owls during the breeding season were
Long-eared Owls (Bezzel et al. 1976), and in northeastern Harz Foreland,
21 Long-eared Owls were found among 612 bird-prey items in pellets of
8-10 breeding pairs of Eagle Owls (Wadewitz & Nicolai 1993). Among
1048 Long-eared Owl ringing recoveries from Finland, Sweden and Russia,
23 were killed by owls or raptors and a further 45 were predated by
unknown animals (ringing recovery printouts from Sweden, Finland and
Russia). Eagle Owls and Goshawks therefore seem to be important predators
of the Long-eared Owl and as such, the degree of predator pressure may
contribute to the pattern of differential migration seen in the last
species. As the female is heavier but without a correspondingly greater
wingspan, it is presumably easier prey for the Eagle Owl and the Goshawk
than the more manoeuvrable male.
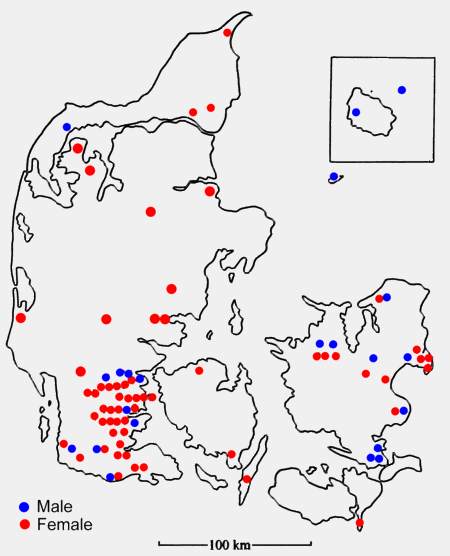
Figure 1: Sex and locality of 82 Long-eared
Owls from Denmark during the winter period. Note the preponderance
of females from Jutland and Funen (48 females: 10 males), compared
with the even sex-ratio on Zealand, Falster, Anholt and Bornholm
(12 females: 12 males).
|
|
Abb. 1: Geschlecht und Fundort von 82 Waldohreulen
gefunden in der Winterperiode in Dänemark. Im Vergleich
zu Jutland und Fünen, wo die Anzahl der weiblichen Exemplare
überwieght (48 weibliche: 10 männliche ), ist der
Unterschied zwischen den Geschlechter auf Seealand, Falster,
Anholt und Bornholm gleich (12 weibliche:12 männliche).
|
 A
prediction arising from our hypthesis is that female Long-eared Owls
will be preyed upon more heavily by Eagle Owls and Goshawks than their
availability relative to male Long-eared Owls would suggest. However,
no information is as yet available on sex determination of predated
Long-eared Owls, not surprising in view of the slight intersexual difference
in skeleton biometrics (Winde 1977). A
prediction arising from our hypthesis is that female Long-eared Owls
will be preyed upon more heavily by Eagle Owls and Goshawks than their
availability relative to male Long-eared Owls would suggest. However,
no information is as yet available on sex determination of predated
Long-eared Owls, not surprising in view of the slight intersexual difference
in skeleton biometrics (Winde 1977).
|
|
Sex
|
|
|
|
|
|
|
Sweden
|
male
|
|
|
|
|
|
|
|
female
|
|
|
|
|
|
|
Denmark
|
male
|
|
|
|
|
|
|
|
female
|
|
|
|
|
|
|
Netherlands
|
male
|
|
|
|
|
|
|
|
female
|
|
|
|
|
|
Table 2: Body mass (g) of Swedish (Malmö
Museum), Danish (pers. obs.), and Dutch (Cramp 1985) Long-eared
Owls. All Swedish and Danish weights are from the non-breeding
period, the Dutch figures from all year round.
|
Tab. 2: Körpermasse schwedischen (Malmö
Museum), dänischen (J.E.) und holländischen Waldohreulen
(Cramp 1985). Alle schwedischen und dänischen Exemplare
stammen aus der Winterperiode, die holländischen vom December
bis März.
|
 To
investigate whether predation pressure by Eagle Owls and Goshawks may
be contributing to the pattern of differential migration seen in the
Long-eared Owl, one approach is to examine the geographical distribution
of all three species. The geographic ranges of Eagle Owls and Goshawks
correspond broadly with that of the Long-eared Owl, although they occur
at lower densities in regions where female biased sex ratios are found:
Denmark, northern Germany, the Netherlands, Belgium, Luxemburg, France
and United Kingdom (Génsbøl 1984, Cramp & Simmons
1980, Cramp 1985). In Germany the strongholds of the Eagle Owl are in
central and southern Germany (Hagemeijer & Blair 1997), areas from
which there are few ringing recoveries of Fenno-Scandian Long-eared
Owls. In Poland, also a stronghold of the Goshawk, there are only 10
recoveries from Fenno-Scandia and Russia out of a total of 1090 (Glutz
von Blotzheim 1980 and ringing recovery printouts from Sweden, Finland
and Russia). It therefore seems likely that migrant owls from Fenno-Scandia
and Russia winter or at least pass through northern Germany, and in
general spend the winter where Eagle Owls occur at low density. An additional
reason for the female to migrate may be that with thick snow cover in
the northern winter it is more difficult for the less manoeuvrable female
to catch voles. Table 2 gives body mass data from Sweden, Denmark and
the Netherlands. The results indicate a trend for body mass to increase
southwards, perhaps indicating a more plentiful food supply in the south,
additional reason for female-dominated migration south. If prey densities
are indeed higher in southern countries, female Long-eared Owls may
be better placed to outcompete both migrant and resident males for food.
The greater difference between male and female body masses in the Netherlands
may reflect the difficulties faced by males in the south of the wintering
range. Norwegian Long-eared Owls appear to be increasing (Scott 1997),
so differential migration does indeed seem a successful strategy for
individuals within this population and it seems probable that natural
selection will operate on this trait. To
investigate whether predation pressure by Eagle Owls and Goshawks may
be contributing to the pattern of differential migration seen in the
Long-eared Owl, one approach is to examine the geographical distribution
of all three species. The geographic ranges of Eagle Owls and Goshawks
correspond broadly with that of the Long-eared Owl, although they occur
at lower densities in regions where female biased sex ratios are found:
Denmark, northern Germany, the Netherlands, Belgium, Luxemburg, France
and United Kingdom (Génsbøl 1984, Cramp & Simmons
1980, Cramp 1985). In Germany the strongholds of the Eagle Owl are in
central and southern Germany (Hagemeijer & Blair 1997), areas from
which there are few ringing recoveries of Fenno-Scandian Long-eared
Owls. In Poland, also a stronghold of the Goshawk, there are only 10
recoveries from Fenno-Scandia and Russia out of a total of 1090 (Glutz
von Blotzheim 1980 and ringing recovery printouts from Sweden, Finland
and Russia). It therefore seems likely that migrant owls from Fenno-Scandia
and Russia winter or at least pass through northern Germany, and in
general spend the winter where Eagle Owls occur at low density. An additional
reason for the female to migrate may be that with thick snow cover in
the northern winter it is more difficult for the less manoeuvrable female
to catch voles. Table 2 gives body mass data from Sweden, Denmark and
the Netherlands. The results indicate a trend for body mass to increase
southwards, perhaps indicating a more plentiful food supply in the south,
additional reason for female-dominated migration south. If prey densities
are indeed higher in southern countries, female Long-eared Owls may
be better placed to outcompete both migrant and resident males for food.
The greater difference between male and female body masses in the Netherlands
may reflect the difficulties faced by males in the south of the wintering
range. Norwegian Long-eared Owls appear to be increasing (Scott 1997),
so differential migration does indeed seem a successful strategy for
individuals within this population and it seems probable that natural
selection will operate on this trait.
 Table
1 indicates that the sex-ratio of wintering Long-eared Owls is female
biased not only in western Denmark but also in the Netherlands and perhaps
northern Germany. However, in most parts of Germany, the sex-ratio was
near parity. The most likely explanation for this is that part of the
wintering population from southern Sweden and Zealand, both with an
equal sex-ratio (cf. Tab. 1), may migrate to more easterly areas in
Germany than migrating birds coming from Jutland where females dominate.
Indeed, ringing recoveries indicate that most birds moving from Jutland
migrate in a southwesterly direction to west Germany, the Netherlands,
Belgium and northern France (ringing recovery printouts). Table
1 indicates that the sex-ratio of wintering Long-eared Owls is female
biased not only in western Denmark but also in the Netherlands and perhaps
northern Germany. However, in most parts of Germany, the sex-ratio was
near parity. The most likely explanation for this is that part of the
wintering population from southern Sweden and Zealand, both with an
equal sex-ratio (cf. Tab. 1), may migrate to more easterly areas in
Germany than migrating birds coming from Jutland where females dominate.
Indeed, ringing recoveries indicate that most birds moving from Jutland
migrate in a southwesterly direction to west Germany, the Netherlands,
Belgium and northern France (ringing recovery printouts).
 In
some avian systems, the larger sex may outcompete the other for food
as nestlings, leading to a biased sex ratio in the population as a whole
(Lack 1954, Yom-Tov & Ollason 1976, both cited in Newton & Marquiss
1979, but see also Newton 1986 and Wiebe & Bertolotti 1992). However,
in parts of Denmark (Zealand) and most of Germany, Long-eared Owl sex-ratios
exhibit parity, with 12 males : 12 females found in Denmark, and 33
males : 31 females in eastern and west-central Germany. A similar pattern
was also found in museum collections in Malmö and Stockholm, which
would seem to be evidence against the above mechanism operating in Long-eared
Owls. In Norway there is a male-biased sex ratio during the winter months,
presumably because many females migrate south (Overskaug & Kristiansen
1994). In
some avian systems, the larger sex may outcompete the other for food
as nestlings, leading to a biased sex ratio in the population as a whole
(Lack 1954, Yom-Tov & Ollason 1976, both cited in Newton & Marquiss
1979, but see also Newton 1986 and Wiebe & Bertolotti 1992). However,
in parts of Denmark (Zealand) and most of Germany, Long-eared Owl sex-ratios
exhibit parity, with 12 males : 12 females found in Denmark, and 33
males : 31 females in eastern and west-central Germany. A similar pattern
was also found in museum collections in Malmö and Stockholm, which
would seem to be evidence against the above mechanism operating in Long-eared
Owls. In Norway there is a male-biased sex ratio during the winter months,
presumably because many females migrate south (Overskaug & Kristiansen
1994).
 The
data presented here seem to support our predation hypothesis - both
Eagle Owls and Goshawks are common in the breeding range of the Long-eared
Owl in Fenno-Scandia, but rare or absent in the winter range, where
female Long-eared Owls predominate. The
data presented here seem to support our predation hypothesis - both
Eagle Owls and Goshawks are common in the breeding range of the Long-eared
Owl in Fenno-Scandia, but rare or absent in the winter range, where
female Long-eared Owls predominate.
5. Zusammenfassung
 Der
Artikel zeigt, dass im westlichen Teil Dänemarks in den Monaten
November bis einschl. Februar bei den Waldohreulen ein zahlenmäßiger
Unterschied der Geschlechter existiert. Während die Geschlechtsverteilung
auf Seeland und den umliegenden Inseln in etwa gleich ist (12 männliche
, 12 weibliche), existiert in Jütland und auf Fünen ein deutlicher
Überwiegen weiblicher Exemplare (48 weibliche , 10 männliche).
Auch für Holland und England wurde diese Ungleichheit nachgewiesen,
dagegen sind in Norwegen im Winter überwiegend männliche Vögel
anzutreffen. Recherchen in den Vogelbalgsammlungen der grösseren
nordeuropäischen Museen bestätigen dieses Resultat. Für
Schweden und Central Europa aber ergibt sich allerdings für die
Wintermonate eine ausgeglichene Geschlechtsverteilung. Der
Artikel zeigt, dass im westlichen Teil Dänemarks in den Monaten
November bis einschl. Februar bei den Waldohreulen ein zahlenmäßiger
Unterschied der Geschlechter existiert. Während die Geschlechtsverteilung
auf Seeland und den umliegenden Inseln in etwa gleich ist (12 männliche
, 12 weibliche), existiert in Jütland und auf Fünen ein deutlicher
Überwiegen weiblicher Exemplare (48 weibliche , 10 männliche).
Auch für Holland und England wurde diese Ungleichheit nachgewiesen,
dagegen sind in Norwegen im Winter überwiegend männliche Vögel
anzutreffen. Recherchen in den Vogelbalgsammlungen der grösseren
nordeuropäischen Museen bestätigen dieses Resultat. Für
Schweden und Central Europa aber ergibt sich allerdings für die
Wintermonate eine ausgeglichene Geschlechtsverteilung.
 Ein
Massenvergleich zwischen schwedischen, dänischen und holländischen
Waldohreulen zeigt, daß die holländischen Exemplare 25 %
schweres sind als die schwedischen und die männlichen Exemplare
17 % weniger wiegen als die weiblichen, wohingegen die schwedischen
und dänischen nur 11-12 % weniger an Körpermasse aufweisen.
Möglicherweise haben die männlichen Eulen in Holland es in
den Wintermonaten besonders schwer, mit der Überzahl weiblicher
Waldohreulen zu konkurrieren. Ein
Massenvergleich zwischen schwedischen, dänischen und holländischen
Waldohreulen zeigt, daß die holländischen Exemplare 25 %
schweres sind als die schwedischen und die männlichen Exemplare
17 % weniger wiegen als die weiblichen, wohingegen die schwedischen
und dänischen nur 11-12 % weniger an Körpermasse aufweisen.
Möglicherweise haben die männlichen Eulen in Holland es in
den Wintermonaten besonders schwer, mit der Überzahl weiblicher
Waldohreulen zu konkurrieren.
 Die
Mehrzahl der Waldohreulen, die über den westlichen Teil Dänemarks
zieht sind junge Weibchen. Keine der bisherigen Hypothesen zur Erklärung
eines unterschiedlichen Zugverhalten der Geschlecter kann diesen Befund
allerdings befriedigend deuten, was die Verfasser zur Formulierung einer
neuen Hypothese veranlasste: Wo eine Population mit sexuellem Dimorphismus
häufig Beute von Prädatoren ( z.B. Habitz und Uhu) wird, wandert
das stärker gefährdete Geschlecht im Winter in Gebiete mit
weniger oder keinen Feinden ab. Die
Mehrzahl der Waldohreulen, die über den westlichen Teil Dänemarks
zieht sind junge Weibchen. Keine der bisherigen Hypothesen zur Erklärung
eines unterschiedlichen Zugverhalten der Geschlecter kann diesen Befund
allerdings befriedigend deuten, was die Verfasser zur Formulierung einer
neuen Hypothese veranlasste: Wo eine Population mit sexuellem Dimorphismus
häufig Beute von Prädatoren ( z.B. Habitz und Uhu) wird, wandert
das stärker gefährdete Geschlecht im Winter in Gebiete mit
weniger oder keinen Feinden ab.
6. REFERENCES
 Berthold,
P. (1984): The control of partial migration in birds, a review. The
Ring. Ser.A. Int. Orn. Bull. 1984 10:253-266. Berthold,
P. (1984): The control of partial migration in birds, a review. The
Ring. Ser.A. Int. Orn. Bull. 1984 10:253-266.
 Bezzel,
E., J. Obst & K-H. Wickl (1976): Zur Ernährung und Nahrungswahl
des Uhus (Bubo bubo). J. Orn. 117:210-238. Bezzel,
E., J. Obst & K-H. Wickl (1976): Zur Ernährung und Nahrungswahl
des Uhus (Bubo bubo). J. Orn. 117:210-238.
 Clark,
R. J., D. G. Smith & L. H. Kelso (1978): Working bibliography of
owls of the world. Natl. Wildlife Fed., Washington, D. C. Clark,
R. J., D. G. Smith & L. H. Kelso (1978): Working bibliography of
owls of the world. Natl. Wildlife Fed., Washington, D. C.
 Cramp,
S., & K. E. L. Simmons (1980): The birds of the Western Palearctic.
Vol. 2. Oxford Univ. Press. Cramp,
S., & K. E. L. Simmons (1980): The birds of the Western Palearctic.
Vol. 2. Oxford Univ. Press.
 Cramp,
S. (1985): The birds of the Western Palearctic.Vol. 6. Oxford University
Press, Oxford. Cramp,
S. (1985): The birds of the Western Palearctic.Vol. 6. Oxford University
Press, Oxford.
 Fowler,
J. & L. Cohen. (no date): Statistics for Ornithologists. BTO Guide
22. British Trust for Ornithology, Thetford, UK. Fowler,
J. & L. Cohen. (no date): Statistics for Ornithologists. BTO Guide
22. British Trust for Ornithology, Thetford, UK.
 Gauthreaux,
S. S. (1978): The ecological significance of behavioural dominance.
In: Perspectives in Ethology. Bateson, P. P. G., & P. H. Klopfer,
(eds.) Vol. 3. pp 17-54. Plenum Publishing Corp., New York. Gauthreaux,
S. S. (1978): The ecological significance of behavioural dominance.
In: Perspectives in Ethology. Bateson, P. P. G., & P. H. Klopfer,
(eds.) Vol. 3. pp 17-54. Plenum Publishing Corp., New York.
 Génsbøl,
B. (1984): Rovfuglene i Europa, Nordafrika og Mellemøsten. Gad,
Copenhagen. Génsbøl,
B. (1984): Rovfuglene i Europa, Nordafrika og Mellemøsten. Gad,
Copenhagen.
 Glutz
von Blotzheim, U. N., & K. M. Bauer (1980). Handbuch der Vögel
Mitteleuropas. Vol. 9. Columbiformes-Piciformes. Akademische Verlagsgesellschaft,
Wiesbaden. Glutz
von Blotzheim, U. N., & K. M. Bauer (1980). Handbuch der Vögel
Mitteleuropas. Vol. 9. Columbiformes-Piciformes. Akademische Verlagsgesellschaft,
Wiesbaden.
 Harvey,
P. V., & N. Riddiford (1990): An uneven sex-ratio of migrant Long-eared
Owls. Ring and Migration 11:132-135. Harvey,
P. V., & N. Riddiford (1990): An uneven sex-ratio of migrant Long-eared
Owls. Ring and Migration 11:132-135.
 Hortling,
I. (1929): Ornitologisk Handbok. J. Simelii Arvingars Boktryckeri, Helsingfors. Hortling,
I. (1929): Ornitologisk Handbok. J. Simelii Arvingars Boktryckeri, Helsingfors.
 Ketterson,
E. D. (1979): Aggressive behaviour in wintering Dark-eyed Juncos: determinants
of dominance and their possible relationship to geographic variation
in sex ratio. Wilson Bull. 91:371-383. Ketterson,
E. D. (1979): Aggressive behaviour in wintering Dark-eyed Juncos: determinants
of dominance and their possible relationship to geographic variation
in sex ratio. Wilson Bull. 91:371-383.
 Ketterson,
E. D., & J. R.King (1977): Metabolic and behavioural responses to
fasting in the White-crowned Sparrow (Zonotrichia leucophrys gambelli).
Physiol. Zool. 50:115-129. Ketterson,
E. D., & J. R.King (1977): Metabolic and behavioural responses to
fasting in the White-crowned Sparrow (Zonotrichia leucophrys gambelli).
Physiol. Zool. 50:115-129.
 Ketterson,
E. D., & V. Nolan, Jr. (1976): Geographic variation and its climatic
correlates in the sex ratio of eastern-wintering Dark-eyed Juncos (Junco
hyemalis hyemalis). Ecology 57: 679-693. Ketterson,
E. D., & V. Nolan, Jr. (1976): Geographic variation and its climatic
correlates in the sex ratio of eastern-wintering Dark-eyed Juncos (Junco
hyemalis hyemalis). Ecology 57: 679-693.
 Ketterson,
E. D., & V.Nolan, Jr. (1979): Seasonal, annual and geographic variation
in sex ratio of wintering populations of Dark-eyed Juncos (Junco
hyemalis). Auk 96:532-536. Ketterson,
E. D., & V.Nolan, Jr. (1979): Seasonal, annual and geographic variation
in sex ratio of wintering populations of Dark-eyed Juncos (Junco
hyemalis). Auk 96:532-536.
 Ketterson,
E. D. & V. Nolan, Jr. (1983): The evolution of differential bird
migration. In: Johnston, R. F. (ed.) Current Ornithology, Vol. 2. Plenum
Press, New York, pp. 357-402. Ketterson,
E. D. & V. Nolan, Jr. (1983): The evolution of differential bird
migration. In: Johnston, R. F. (ed.) Current Ornithology, Vol. 2. Plenum
Press, New York, pp. 357-402.
 Kjellén,
N. (1994): Differences in age and sex ratio among migrating and wintering
raptors in southern Sweden. Auk 111 (2):274-84. Kjellén,
N. (1994): Differences in age and sex ratio among migrating and wintering
raptors in southern Sweden. Auk 111 (2):274-84.
 Lack,
D. (1966): Population studies in Birds. Clarendon Press, Oxford. Lack,
D. (1966): Population studies in Birds. Clarendon Press, Oxford.
 Lundberg,
A. (1979): Residency, migration and a compromise: adaptations to
nest-site scarcity and food specialization in three Fennoscandian owl
species. Oecologia (Berlin) 41:273-281. Lundberg,
A. (1979): Residency, migration and a compromise: adaptations to
nest-site scarcity and food specialization in three Fennoscandian owl
species. Oecologia (Berlin) 41:273-281.
 Lundberg,
A. (1986): Adaptive advantages of reversed sexual size dimorphism in
European owls. Ornis Scandinavica 17:133-140. Lundberg,
A. (1986): Adaptive advantages of reversed sexual size dimorphism in
European owls. Ornis Scandinavica 17:133-140.
 Lundberg,
P. (1988): The evolution of partial migration in birds. Tree 3 no. 7:172-175. Lundberg,
P. (1988): The evolution of partial migration in birds. Tree 3 no. 7:172-175.
 Mikkola,
H. (1983): Owls of Europe. Poyser, Calton. Mikkola,
H. (1983): Owls of Europe. Poyser, Calton.
 Moritz,
D., & E. Schonart (1976): Bemerkenswertes über die Vogelwelt
Helgolands im Jahr 1975. Vogelwelt 8: 107-118. Moritz,
D., & E. Schonart (1976): Bemerkenswertes über die Vogelwelt
Helgolands im Jahr 1975. Vogelwelt 8: 107-118.
 Myers,
J. P. (1981): A test of three hypotheses for latitudinal segregation
of the sexes in wintering birds. Canadian J. Zool. 59:1527-1534. Myers,
J. P. (1981): A test of three hypotheses for latitudinal segregation
of the sexes in wintering birds. Canadian J. Zool. 59:1527-1534.
 Newton,
I. (1986): The Sparrowhawk. T. & A. D. Poyser, Calton. Newton,
I. (1986): The Sparrowhawk. T. & A. D. Poyser, Calton.
 Newton,
I., & M. Marquiss (1979): Sex ratio among nestlings of the European
sparrowhawk. The American Naturalist 113:309-315. Newton,
I., & M. Marquiss (1979): Sex ratio among nestlings of the European
sparrowhawk. The American Naturalist 113:309-315.
 Olsen,
K. M. (1992): Danmarks Fugle - en oversigt. Dansk Orn. Forening, Copenhagen. Olsen,
K. M. (1992): Danmarks Fugle - en oversigt. Dansk Orn. Forening, Copenhagen.
 Overskaug,
K., & E. Kristiansen (1994): Sex ratio of accidentially killed Long-eared
Owls (Asio otus) in Norway. Ring and Migration 15:104-106. Overskaug,
K., & E. Kristiansen (1994): Sex ratio of accidentially killed Long-eared
Owls (Asio otus) in Norway. Ring and Migration 15:104-106.
 Piechocki,
R. (1979): Makroskopische Präparationstechnik. Gustav Fischer Verlag,
Stuttgart. Piechocki,
R. (1979): Makroskopische Präparationstechnik. Gustav Fischer Verlag,
Stuttgart.
 Scott,
D. (1997): The Long-eared Owl. The Hawk and Owl Trust, London. Scott,
D. (1997): The Long-eared Owl. The Hawk and Owl Trust, London.
 Tucker,
G. M. & M. F. Heath (1994): Birds of Europe. Their Conservation
Status.Cambridge, U. K.: BirdLife International (BirdLife Conservation
Series no. 3). Tucker,
G. M. & M. F. Heath (1994): Birds of Europe. Their Conservation
Status.Cambridge, U. K.: BirdLife International (BirdLife Conservation
Series no. 3).
 Uttendörfer,
O. (1939): Die Ernährung der deutschen Raubvögel und Eulen.
Neudamm. Uttendörfer,
O. (1939): Die Ernährung der deutschen Raubvögel und Eulen.
Neudamm.
 Wadewitz,
M. & B. Nicolai (1993): Nahrungswahl des Uhus (Bubo bubo)
im nordöstlichen Harzvorland. Orn. Jber. Mus. Heineanum 11: 91-106. Wadewitz,
M. & B. Nicolai (1993): Nahrungswahl des Uhus (Bubo bubo)
im nordöstlichen Harzvorland. Orn. Jber. Mus. Heineanum 11: 91-106.
 Wiebe,
K. L., & G. R. Bortolotti (1992): Facultative sex ratio manipulation
in American kestrels. Behav. Ecol. Sociobiol. 30: 379-386. Wiebe,
K. L., & G. R. Bortolotti (1992): Facultative sex ratio manipulation
in American kestrels. Behav. Ecol. Sociobiol. 30: 379-386.
 Wijnandts,
H. (1984): Ecological energetics of the Long-eared Owl (Asio otus).
Ardea 72:1-92. Wijnandts,
H. (1984): Ecological energetics of the Long-eared Owl (Asio otus).
Ardea 72:1-92.
 Winde,
H. (1977): Vergleichende Untersuchungen über Proportionalität
und Sexualdimorphismus im Skelett von Asio otus otus (L.). Zool.
Abhandl. 34: 143- 146. Winde,
H. (1977): Vergleichende Untersuchungen über Proportionalität
und Sexualdimorphismus im Skelett von Asio otus otus (L.). Zool.
Abhandl. 34: 143- 146.
 Wyllie,
I., L. Dale & I. Newton (1996): Unequal sex-ratio, mortality causes
and pollutant residues in Long-eared Owls in Britain. Brit. Birds 89:429-436. Wyllie,
I., L. Dale & I. Newton (1996): Unequal sex-ratio, mortality causes
and pollutant residues in Long-eared Owls in Britain. Brit. Birds 89:429-436.
Copyright: Johannes Erritzoe & Richard Fuller, 1999
|
